Despite the fact that manganese technology has been used for thousands of years, there are still some challenges in the industry. There are many factors that contribute to the difficulties involved. For example, the high vapor pressure that manganese can experience makes it difficult to manufacture and control. In order to overcome these challenges, manufacturers must implement new technologies that can improve the quality and performance of manganese.
Characteristics of manganese ore
Despite the fact that there are many different types of manganese ores, they have some common features. These features include the presence of the mineral pyrolusite and the presence of carbonate minerals, such as calcite and magnetite. The mineral psilomelane is usually found in the matrix of manganese ore, while the mineral braunite is normally found as an alteration mineral. Psilomelane can be found in a wide range of colors, from yellowish gray to grayish white. Its botryoidal appearance gives it a granular look. In addition to being used as an alteration mineral, it is also commonly found in thin-section studies.
Manganese is one of the most important elements in the Earth’s crust. It is essential for human and animal systems to function. It is commonly used in steel making, but is used less in the production of batteries. It is used in the production of various non-metallurgical products, including animal feed, brick, and colorants.
Manganese is found as oxides, and occurs in sedimentary rocks. Its chemical properties are similar to those of iron. It is oxidized like iron when water with dissolved oxygen is present. It is also often found in hydrothermally altered rocks. In these rocks, it is commonly present in a +3 valence. It is used in the production of steel because it adds to the stiffness and durability of steel. It can be found in many different forms, including manganese oxide, manganese carbonate, and manganese silicate.
The main types of manganese deposits are sedimentary manganese ore, hydrothermal manganese ore, and supergene manganese ore. These deposits can be produced by a variety of processes, including hydrothermal sedimentation, chemical weathering, and diagenetic processes. The most common type of manganese deposits are sedimentary manganese ore. In these deposits, manganese can be found in a variety of carbonate rocks, including black shales and mudrocks. The chemical composition of manganese ores can vary greatly depending on the processes that produce them. The chemical composition of manganese ore deposits can be determined using various techniques. For example, it is possible to determine the origin of manganese ores by using geochemical analysis. In this study, the mineralogy and the geochemical characteristics of manganese deposits were evaluated from the samples in the Bela ophiolite complex.
The manganese ores in the Bela ophiolite complex were obducted by the Indian-Eurasian continental plates. The manganese ores in this complex were probably formed by hydrothermal solutions that were produced near a sea floor spreading center. The seafloor spreading centers are centers that offer deeper fractures for hydrothermal solutions. The manganese ores in the Bela ophiolite complex belong to the diagenetic-type of manganese deposits. The diagenetic-type of manganese deposits are formed by the processes of hydrothermal sedimentation.
The chemical composition of manganese ores in the Bela ore is described below. The trace elements are listed separately. In addition to manganese, trace elements such as Cu, Ni, and Zn can be detected in the ores. The elements that show the most clear correlations with total iron in black shales are Zn, Mo, and U. The manganese ores also show positive correlations with Ba and Rb.
XRD analysis of manganese oxides with high valence to MnO
XRD analysis of manganese oxides with high valence to MnO is a useful way to obtain geochemical insights into the mineral and its environment. Manganese oxide minerals are found in a variety of environments including oceans, fresh water, marine and soil nodules, and in extended terrestrial depths. Mn oxide minerals are known to play important roles in chemical, redox-related, and water transport processes. They have also been used for thousands of years in a variety of applications, including glass clarification, catalysts, and as a battery material. However, the precise atomic structure of many Mn oxide minerals is not well understood, making it difficult to understand their geochemical behavior.
Manganese oxides are poorly crystalline, and their atomic structure is complex and therefore difficult to understand. Although they have been studied for many years, their crystal structures have not been fully understood. In the past, researchers have used hydrated Na+ ions as structure directors. In recent years, XRD has become a valuable tool for measuring the atomic structures of a variety of materials, including manganese oxides. XRD analysis has provided new insights into the atomic structure of manganese, and these studies have been used to refine structural models based on powder diffraction data.
XRD analysis of manganese oxides has shown that manganite is a redox-sensitive mineral, and it undergoes a series of chemical reactions. During its early stage, manganite consists of Mn3+ cations and structural hydroxyl groups. As it is exposed to oxidizing conditions, it transforms into hausmannite, accompanied by the release of water molecules. This transformation is accompanied by partial reduction of Mn3+ to Mn2+.
In situ synchrotron XRD studies have been used to better understand manganese oxides and to control the structure of these materials. For example, a Na-birnessite-derived manganese oxide has been synthesized and characterized. This manganese oxide is characterized by a Na+2 x 3 tunnel structure. The manganese oxide has been analyzed by XRD and the results showed that the Na+ cations are distributed around the gasket rims of the manganite sample. The structure of this manganese oxide is in good agreement with the romanechite pattern.
XRD analysis of manganese dioxides also has shown that these particles are single-crystalline. They exhibit urchinlike architectures. They also have a peak intensity that increases with the amount of dopant. This peak intensity is proportional to the initial valence of Mn centers. The crystalline structure of manganese dioxides can be tailored, making them attractive catalysts. The presence of gold nanoparticles in manganese oxides can significantly enhance their catalytic activity. XRD analysis of manganese oxides can also be used to determine the oxidation state of manganese. In He, two reduction peaks are observed, suggesting that manganese activates oxidation sites. These findings suggest that manganese dioxides may contain chromium cations.
Application of manganese in batteries
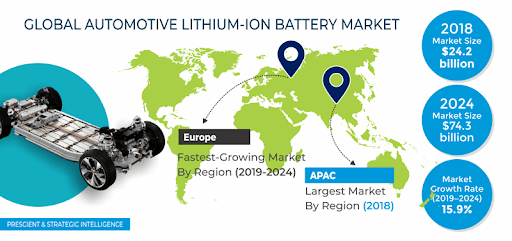
Increasingly, manganese is being used in battery technology. It plays a vital role in steel, stainless steel and electric vehicle (EV) batteries. It is also used in a wide variety of other industries. Moreover, it plays a crucial role in the global climate change and renewable energy sectors. Its demand is also expected to rise in the near future.
Several companies are focusing on manganese technology in batteries. For instance, Maxtech Ventures has recently secured a large number of high-grade manganese claims in Brazil. The company is also planning to increase its resource capacity to cater to the growing power cell market. In addition, it has signed a five-year research agreement with NMC battery researcher Dr. Jeff Dahn. The agreement will help the company to bring down costs and develop NMC battery technology.
In addition, manganese is used as an alternative to cobalt in lithium-ion battery cathode materials. In a recent study, the University of Waterloo showed that manganese dioxide nanosheet can improve the performance of lithium-sulphur batteries. This technology could increase the range of electric vehicles by up to three times. Other major car makers are also interested in adopting the technology.
Manganese is a hard, brittle metal. It is very reactive when pure and is oxidized quickly. It is also reactive with water. When dissolved in water, manganese salts can act as aqueous electrolytes. It also has high redox activity and is useful as a cathode material.
Manganese is also used in nickel-metal hydride batteries, which are used in hybrid vehicles such as the Toyota Prius. It is also used in sodium-ion batteries for stationary storage applications. But the current investment in manganese mining infrastructure might lag behind the current demand for manganese.
As with other battery chemistries, manganese technology in batteries is not new. It is already being used in lithium-ion battery cathodes, which are the most common type. It is also used in traditional layered lithium-ion battery cathodes. In fact, manganese is slightly overtaking nickel in terms of battery market share.
As lithium-ion batteries become more popular, the demand for manganese chemistries is expected to rise. Moreover, as the government’s policies shift towards cleaner fuels and EVs, it is likely that the demand for manganese will increase. As a result, the price of manganese could also rise.
Currently, the US is 100% dependent on manganese imports. This situation is similar to other industrialized countries. However, the recent investment in manganese mining infrastructure could lead to steep price increases. It is therefore important for companies to address the demand gap. This is why Maxtech Ventures is focusing on building a vertical mine and selling manganese into high growth markets. It has also secured a joint exploration agreement with Maringa Ferro-Liga S.A., a company in Brazil, to explore manganese-specific projects.

An author of Update UI, We have published more articles focused on blogging, business, lifestyle, digital marketing, social media, web design & development, e-commerce, finance, health, SEO, travel.
For any types of queries, contact us on updateui.info@gmail.com.